Part 2:
A Review of Adiabatic Processes
Knowing the rate at which rising air cools is vital in determining the
stability of the atmosphere. We have briefly introduced dry, moist, and
saturated adiabatic processes in previous sessions, but because these
concepts are so important to the discussion on atmospheric stability, we
will take a few minutes to review them, as well as introduce a couple of
others that are of equal importance.
The Dry Adiabatic Process
When a parcel of air rises, it expands, and the temperature decreases.
Likewise, when air sinks, it compresses, and the temperature increases.
This phenomenon occurs without adding or withdrawing energy from the parcel
and is illustrated in Session 3 by the equation of state and Poisson's equation. When a parcel of
air, either dry or containing water vapor, rises or sinks without the addition or
extraction of heat, that process is said to be a dry adiabatic
process. Even though a parcel of air may contain moisture, if the parcel
is rising, then it cools according to the dry adiabatic lapse rate
until it reaches the dew point temperature
(Td).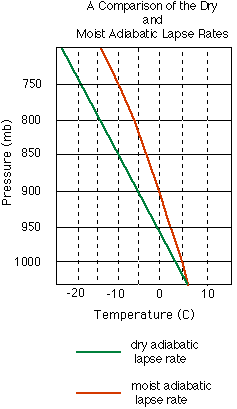 (Note: The dew point temperature for a
rising air parcel is not equal to the dew point temperature of the same
parcel at the surface. As the parcel rises, the dew point temperature
decreases slightly in response to the decrease in pressure.) We refer to
the pressure where the actual temperature equals the dew point temperature
as the Lifting Condensation Level
(LCL). At the LCL, the cooling process becomes a moist or saturated
adiabatic process.
(Note: The dew point temperature for a
rising air parcel is not equal to the dew point temperature of the same
parcel at the surface. As the parcel rises, the dew point temperature
decreases slightly in response to the decrease in pressure.) We refer to
the pressure where the actual temperature equals the dew point temperature
as the Lifting Condensation Level
(LCL). At the LCL, the cooling process becomes a moist or saturated
adiabatic process.
Moist Adiabatic Process
The amount of water vapor a sample of air can hold is dependent on the
temperature and pressure of the sample. As a parcel of air cools,
its relative humidity
increases, provided no moisture is either added or removed from the
parcel. In general, as a sample cools, its capacity to hold water
decreases. When the air reaches a point of saturation, condensation
begins. As the water vapor condenses it goes from a higher energy state to
a lower one, and as a result, latent heat is released into the air.
In an effort to simplify the process, meteorologists assume that an air
parcel is bounded by an imaginary balloon-like skin that contains the
parcel and does not allow any mixing with its surrounding environment.
Therefore, any latent heat released by condensation is contained in the
parcel and is utilized to heat only the sample parcel. So, for the case of
a rising, expanding, saturated parcel of air, the only heat added to this
theoretically closed system is the heat generated within the parcel
itself. The energy of the parcel is said to be conserved because the
latent heat is a conversion from existing energy and not energy that was
added to the system.
If a rising saturated parcel suddenly changes directions and starts to
sink (compression), then evaporation of the condensation products will consume the
latent heat to re-form water vapor. Since no heat is exchanged between the
parcel and the environment, we still refer to this heating and cooling as
an adiabatic process. However, during the processes of condensation and
evaporation, the cooling and heating of the saturated parcel varies somewhat from the purely dry adiabatic process we discussed above. A rising saturated parcel cools at a slower rate due to the release of latent heat, and a sinking saturated parcel heats more slowly due to the conversion of heat energy during evaporation. This cooling and heating of a rising and sinking saturated parcel is called a moist or saturated adiabatic
process.
The Pseudoadiabatic Process
We have covered the cases of dry air, unsaturated moist air, and saturated
air, so what could possibly be left? Well, we all know from personal
experience that if condensation continues long enough we get rain. We
will see in Session 8 that the process of forming a rain drop is more
complex than just simple condensation. But for our purposes here, the
point is that all that condensing moisture does not remain in the cloud
just to be evaporated at some later time. So, we must address the case
where the moisture precipitates out of the cloud.
If you remember our discussion of temperature in
Session 3, all substances are composed of molecules in motion which have
kinetic energy. As rain, snow, or any other form of precipitation falls
out of a cloud, it carries with it that energy it possesses. With this
true loss of energy, the process is no longer adiabatic and, therefore, is
called pseudoadiabatic. Fortunately, the amount of energy lost
through precipitation is very small compared to the energy of the air
molecules. The pseudoadiabatic lapse rate is so close to the moist
adiabatic lapse rate that meteorologists tend to ignore this difference
and often refer to them synonymously. In fact, on adiabatic charts (or
pseudoadiabatic charts), the moist adiabatic lapse rate lines are referred
to interchangeably as moist, saturated, or pseudoadiabatic.



Developed by
 The Shodor
Education Foundation, Inc.
The Shodor
Education Foundation, Inc.
Copyright © 1996
 (Note: The dew point temperature for a
rising air parcel is not equal to the dew point temperature of the same
parcel at the surface. As the parcel rises, the dew point temperature
decreases slightly in response to the decrease in pressure.) We refer to
the pressure where the actual temperature equals the dew point temperature
as the Lifting Condensation Level
(LCL). At the LCL, the cooling process becomes a moist or saturated
adiabatic process.
(Note: The dew point temperature for a
rising air parcel is not equal to the dew point temperature of the same
parcel at the surface. As the parcel rises, the dew point temperature
decreases slightly in response to the decrease in pressure.) We refer to
the pressure where the actual temperature equals the dew point temperature
as the Lifting Condensation Level
(LCL). At the LCL, the cooling process becomes a moist or saturated
adiabatic process. The Shodor
Education Foundation, Inc.
The Shodor
Education Foundation, Inc.