Course Chapters
Section Tests
Online Calculators Linear Least Squares Regression Newton's Method Equation Solver
Related Information Links
|
Problem 2 Solution(a) 4689 atoms (b) 475 atoms Solution Steps for Part (a):If a sample of ammonia contains 1563 nitrogen atoms, how much hydrogen is present?
Answer:We know the ratio of nitrogen to hydrogen atoms is 1 : 3, so we can set up the proportion:
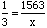 Where x is the (unknown) amount of hydrogen atoms. Cross multiplying gives x = 1563 * 3 or x = 4689. Solution Steps for Part (b):If a sample contains 1425 hydrogen atoms, how much nitrogen is present?
Answer:We know the ratio of nitrogen to hydrogen atoms is 1 : 3, so we can set up the proportion: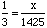 Cross multiplying gives 1425 = 3 x or (dividing by 3 on a calculator) x = 475. |
 Shodor
Shodorin cooperation with the Department of Chemistry,
The University of North Carolina at Chapel Hill
Copyright © 1996-2008 Shodor
Please direct questions and comments about this page to
[email protected]