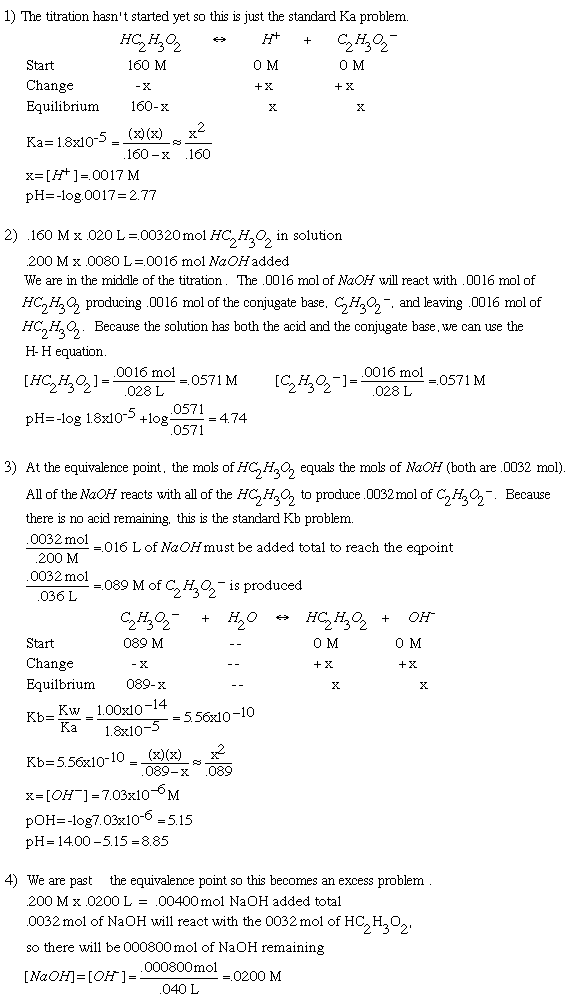
Course Chapters
Section Tests
Online Calculators Linear Least Squares Regression Newton's Method Equation Solver
Related Information Links
|
Titration Solution20.00 mL of 0.160 M HC2H3O2 (Ka=1.8x10-5) is titrated with .200 M NaOH.1. What is the pH of the solution before the titration begins? 2. What is the pH after 8.00 mL of NaOH has been added? 3. What is the pH at the equivalence point? 4. What is the pH after 20.00 mL of NaOH has been added?
Back to the Acid Base Page |
 Shodor
Shodorin cooperation with the Department of Chemistry,
The University of North Carolina at Chapel Hill
Copyright © 1996-2008 Shodor
Please direct questions and comments about this page to
[email protected]