Course Chapters
Section Tests
Online Calculators Linear Least Squares Regression Newton's Method Equation Solver
Related Information Links
|
Balancing EquationsThe trick with this one is to treat the phosphate ion (PO4-3) as an element, and not break it into two individual elements.If you do that, you get:
Fe: 1a + 0b -3c = 0d Note that you do not have a square matrix for Matrix A. Following the regular procedures, you should get a 4 x 3 matrix (four rows, three columns). You can make it square without changing the algorithm by adding a column of 1's at the end of the matrix.
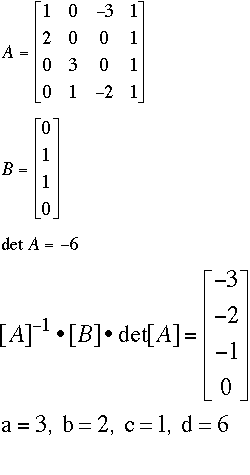
Go to: Back to Balancing Equations, Mathematics Index, |
 Shodor
Shodorin cooperation with the Department of Chemistry,
The University of North Carolina at Chapel Hill
Copyright © 1996-2008 Shodor
Please direct questions and comments about this page to
[email protected]