(click image to view in separate window)

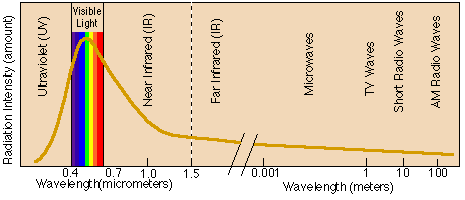
Wavelengths are measured in units of micrometers, because of the small distance between respective wave crests. The sun emits radiation on many wavelengths, but its highest intensity of energy is emitted at wavelengths from 0.4 to 0.7 micrometers. Luckily, this is the band we know as visible radiation or light (if we saw at very different wavelengths, objects would not be as distinguishable because the reflecting radiation would be significantly less). Energy with wavelengths smaller than 0.4 micrometers is known as ultraviolet, or UV radiation. Energy with wavelengths larger than 0.7 micrometers is known as infrared, or IR, radiation. Humans can see neither UV nor IR radiation. However, the radiation we feel as heat is IR radiation. The only beings that can see UV and IR radiation are aliens or cool people on Star Trek . Sometimes, you might hear about military people using IR goggles to see in total darkness. The goggles do not actually "see" IR radiation, but interpret IR radiation and present it in a band of visible radiation that humans can see.
Because the sun is very hot (approximately 6000 Kelvin (K), which is about 10000°F), it emits radiation at very short wavelengths, most of it less than 2 micrometers. In contrast, the earth has an average temperature of around 25 degrees C (288 K, 77°F). Therefore, the earth emits radiation of much longer wavelengths, generally between 5 and 25 micrometers. Thus, solar radiation is known as shortwave radiation, and terrestrial radiation (that from the earth) is known as longwave radiation. These two categories are used widely when discussing heat balances (in next session). There are, of course, many other forms of radiation, some with wavelengths on the order of 100 or so meters. A list of common radiations and their respective wavelengths are presented below.
| Type of Radiation | Approximate Wavelength |
|---|---|
| AM radio waves | 100 meters |
| Television waves | 1 meter |
| Microwaves | 1 millimeter |
| Infrared waves | 1 micrometer |
| Visible light | 0.3 to 0.7 micrometers |
| Ultraviolet waves | 0.1 micrometers |
| X rays | 0.001 micrometers |
| Surface | Albedo (%) | Surface | Albedo (%) |
|---|---|---|---|
| Snow | 79-95 | Dark Soil | 5-15 |
| Ice | 30-40 | Grassy Field | 10-30 |
| Thick Clouds | 60-90 | Forest | 5-15 |
| Thin Clouds | 30-50 | Water | 10 (avg.) |
| Wet Sand | 20-30 | Venus | 78 |
| Dry Sand | 35-45 | Mars | 17 |
| Concrete | 17-27 | Moon | 7 |
| Asphalt | 5-10 | Earth | 34-42 |
 The Shodor
Education Foundation, Inc.
The Shodor
Education Foundation, Inc.