Part 3:
Factors Influencing Energy Balance
We have mentioned that different surfaces absorb and emit radiation at
different intensities and wavelengths. The atmosphere and earth's surface
are composed of countless substances, each with its own energy properties.
In this section, we discuss how some of the more important factors and
substances affect energy balance in the atmosphere. These areas include
ground cover, cloud cover, greenhouse gases, and pollution.
Sunlight (shortwave radiation) hits the surface of the earth, and is
converted into heat. However, the earth's surface is made up of highly
varied areas each with their own radiation absorption and emission
characteristics. For instance, a forest does not absorb or release heat as
quickly as asphalt on a highway. The heated air on the highway will rise
more quickly than air in the forest. This may seem obvious to some, but
the details are very important when looking at the specifics of turbulence
in the atmosphere. In general, darker surfaces absorb and emit energy more
quickly than lighter surfaces. Lighter surfaces tend to reflect more
energy and absorb less of it (see albedo
table).
Water is another surface of great importance. You may have noticed that
air temperatures near major bodies of water seem warmer in the winter and
cooler in the summer. That is because water absorbs and emits radiation
much slower than land. Water also has a much greater heat capacity
than the land. The heat capacity of a substance is the ratio of the
amount of heat absorbed by the substance to the amount of temperature rise.
The specific heat of a substance is the amount of energy
required to raise the temperature of one unit of mass of the substance one
degree. Pure water, with an initial temperature of 15 C, has a specific
heat of one cal/g for a change of one degree Celsius. The specific
heat of sandy clay is only 0.33 cal/g x C. Though a body of water may
receive intense summer sunlight, it will warm much slower than the land
near it. If the winds are blowing onshore, the cooler air above the water
can be mixed with the warmer air over land, allowing the surrounding air
to cool. The opposite is true in the winter. In the same way the water
warms more slowly than the land, the water also cools more slowly. The warmer air from above the water mixes with the surrounding air
to warm the land. This often occurs on a daily basis, forming what we call
the sea breeze and land breeze (these are discussed in Session 5). Also,
water goes through evapotranspiration
processes that impact the local energy balance near the surface. Water
(moisture) also has an impact on the energy balance in the atmosphere,
especially when it is visible, in the form of clouds.
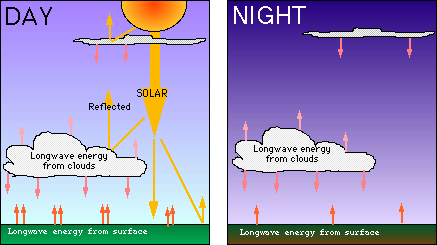
Clouds play an important part in energy balance. Clouds both reflect and
release radiation efficiently. Shortwave radiation from the sun is
efficiently reflected, and longwave radiation from the earth is
efficiently absorbed and emitted. High, thin clouds radiate heat (longwave
radiation) back towards the earth, whereas low, usually thicker clouds,
reflect incoming sunlight, but also absorb and emit heat from the surface.
That is why in the summer, very cloudy days seem cooler than clear days,
and cloudy nights seem warmer than clear nights. The clouds act as
trapping agents, keeping the surface heat in, but not allowing much more
to enter or escape (see diagram below).
As mentioned earlier, greenhouse gases trap heat and emit it very
efficiently. This plays a crucial, but complicated role in energy balance.
As greenhouse gases increase in concentration, the air will gradually
warm. The warmer air will cause more surface water to evaporate, which may
lead to the formation of more clouds. But these clouds could prevent
sunlight from warming the earth. So although greenhouse gases may lead to
global warming, they may also lead to more clouds, possibly negating the
warming effects. This is but one interaction involved in the complex issue
of energy balancing, one we do not fully understand.
Airborne pollutants also affect energy balance. Pollutants in the
atmosphere react with other natural and anthropogenic chemicals present,
sometimes releasing heat energy, sometimes absorbing heat energy. Some
pollutants also play a role in the greenhouse effect. Atmospheric
chemistry is an entire field of study, which we encourage you to learn
more about by looking at our companion course in Computational
Atmospheric Science on-line. Though the scope of this course will
allow us to mention it only briefly here, we emphasize that chemistry
plays an important role in energy balance in the atmosphere.





 The Shodor
Education Foundation, Inc.
The Shodor
Education Foundation, Inc.