In Categorization of Aerosols and Their Sources, at the beginning of this session, we defined three categories of aerosols. These categories grouped aerosols ranged less than 0.2 micrometers to more than 2 micrometers in diameter. From the table below, a typical cloud condensation nucleus (CCN) is about 0.2 micrometers in diameter, while a typical raindrop is about 2000 micrometers or 2 mm in diameter. The increase in size from a CCN to a raindrop represents a growth of 10,000 times the initial size of the CCN.
| Droplet or Nuclei | Average Diameter (micrometers) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Typical CCN | 0.2 | Typical Cloud Droplet | 20 | Large Cloud Droplet | 100 | Typical Raindrop | 2000 |
There are two growth processes that any droplet that grows beyond
about 20 micrometers in diameter will experience: the
diffusion process and the collision-coalescence
process. We will briefly discuss each of these next.

The Diffusion Process
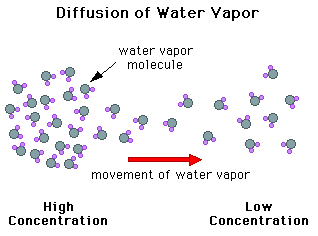
The continued growth of a cloud droplet, once condensation has started, is initially governed by the diffusion of the water vapor molecules toward the droplet. Diffusion is the process of molecules moving from regions of higher concentrations to regions of lower concentrations. At the surface of a droplet, water vapor is simultaneously condensing and evaporating. When the concentration of water vapor molecules is higher some distance from the droplet than it is at the droplet surface, the water vapor in the air diffuses toward the droplet, condenses onto the droplet, and the net effect is droplet growth. Two phenomena which influence the growth that occurs by diffusion are the curvature effect and the solution effect.
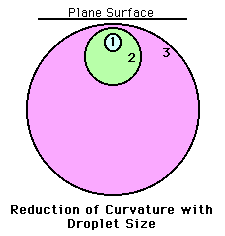 Droplets, by nature, are round. The curvature of a droplet tends
to increase the concentration of vapor at the surface of the
droplet. Small droplets have more curvature than larger droplets.
In general, given identical atmospheric conditions, a smaller
droplet will have a greater concentration of water vapor at its
surface than a larger droplet. Since diffusion is the movement
from higher concentrations to lower concentrations, the curvature
effect tends to retard droplet growth by diffusion.
As a droplet grows, its curvature decreases and becomes more like
a plane surface and the influence of the curvature effect
decreases as well.
Droplets, by nature, are round. The curvature of a droplet tends
to increase the concentration of vapor at the surface of the
droplet. Small droplets have more curvature than larger droplets.
In general, given identical atmospheric conditions, a smaller
droplet will have a greater concentration of water vapor at its
surface than a larger droplet. Since diffusion is the movement
from higher concentrations to lower concentrations, the curvature
effect tends to retard droplet growth by diffusion.
As a droplet grows, its curvature decreases and becomes more like
a plane surface and the influence of the curvature effect
decreases as well.
In contrast with the curvature effect, the solution effect encourages growth by diffusion. Many aerosols dissolve in water. As water condenses onto them, the combination of the aerosol and water create a solution droplet. The dissolved substance displaces some of the water molecules at the surface of the droplet, and the result is a decreased concentration of water vapor at the surface. In general, the more concentrated the droplet solution is, the less concentrated the water vapor at the surface of the droplet will be. Thus, the solution effect tends to positively influence growth by diffusion. The solution effect can often encourage growth at relative humidities below 100%. As a solution droplet grows, the concentration of the droplet is diluted and the influence of the solution effect also decreases.
The curvature and solution effects work in opposition to one
another on a backdrop of fluctuating atmospheric vapor pressure.
A droplet that is neither growing nor decaying (i.e., getting
smaller) is in equilibrium with the surrounding air. This means
the concentration of water vapor at the droplet surface is equal
to the concentration of water vapor a short distance away from
the droplet. If the relative humidity increases for any reason,
a droplet in equilibrium will grow until it depletes the air of
excess water vapor and reaches equilibrium once again.
Conversely, if the relative humidity decreases, the droplet will
evaporate until it reaches equilibrium. A droplet that manages
to grow to a diameter of about 20 micrometers will start to grow
by collision and coalescence.
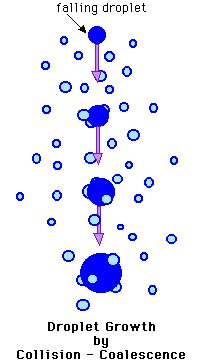
The Collision-Coalescence
Process
A droplet may continue to grow by diffusion beyond 20 micrometers
in diameter, however, once a droplet attains this size, growth is
slow and inefficient. Droplets this large begin to collide and
coalesce with other droplets as they fall through the cloud,
meaning they will bump into and bond to one another and form
larger drops. Updrafts in a cloud can transport a droplet upward
repeatedly allowing it many opportunities to fall back down
through the cloud and collide and coalesce with other droplets.
Initially by diffusion, and subsequently by collision and
coalescence, tiny aerosol nuclei grow into large water droplets
more than 10,000 times their initial size.
 The
Shodor
Education Foundation, Inc.
The
Shodor
Education Foundation, Inc.