Part 2:
Vertical Structure of the Atmosphere
Imagine yourself in a balloon, traveling from the Earth's surface upwards
through the atmosphere. As you rise, you will notice decreases in air
density and air pressure. You may be surprised to discover that you will
also feel both decreases and increases in air temperature. These changes
are associated with distinct layers in the atmosphere, each with
individual properties and characteristics. We will address each of these
changes in turn, as well as the distinctions of each layer.
Density is more or less a measure of concentration. It is expressed in
terms of mass per unit volume. In a comparison of solids, liquids, and gases to one another, solids are the most dense while gases are the least dense. Solids have many more molecules (thus, more mass) per unit volume than either liquids or gases. Molecules in gases move very freely. In our atmosphere, density decreases rapidly with height (i.e., number of molecules which make up the air decreases with height). This is due to the Earth's gravitational pull. Molecules which are in the atmosphere are pulled towards the center of the earth. Therefore, there are higher concentrations of molecules near
the surface of the earth than 10 miles (16 km) up. In fact, over 90
percent of all molecules in the atmosphere are within the first 10 miles
(16 km). So why do all the molecules not form a layer of uniform
concentration and density near the earth's surface? Well, there is a lot
of mixing and vertical motion that keeps all those molecules moving
around, which we will talk about in later lessons. The dramatic decrease
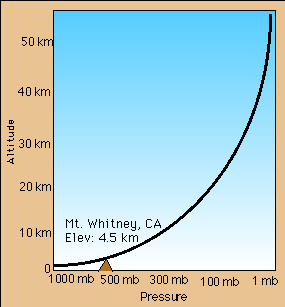
in density as you go up affects the air pressure, causing it to decrease
at a similar rate.
The molecules in the atmosphere are in constant motion. While moving, they
bump into each other and other objects on the order of 10 billion times a
second. Each collision exerts a small force on the other object, creating
a pressure. As the concentration of molecules decreases, so do the number
of collisions and therefore, the associated pressure. Because the density
of the atmosphere decreases as you go up, air pressure decreases in the
same proportion.
There's another important part to the concept of pressure. As said above,
molecules in the atmosphere are being pulled toward the earth by gravity.
This pulling is a force also, and so a pressure is exerted at the surface
of the earth. This force can be thought of as the weight of all the air
above any point on the earth's surface. Air is pretty heavy -- 14.7
pounds per square inch (1013.25 millibars or 101.325 kiloPascals) at sea level. (Meteorologists normally refer to pressure in units of either millibars (mb) or kiloPascals (kPa). One millibar is equal to 100 Pascals (Pa) or 0.1 kiloPascals (kPa). The Pascal is the international unit of pressure.)
Ultimately, air pressure is a measure
of all the air above any point, influenced by the molecules' forces of
motion and gravity. It is also important to understand
that the pressure at any given point changes with time because the air
molecules do not stay in the same location. This changing pressure is key
to our concept of weather. Generally, as pressure decreases, the weather
becomes more stormy.
Air temperature also changes with height. Since the number of molecules
decreases with height, it is sometimes assumed that temperature also
decreases with height. This, however, is not always the case. Each layer
in the atmosphere has its own temperature profile. For our purposes, we
will talk about temperature within the descriptions of each layer. But
before we go into layers, we should talk about the importances of sea
level.
The earth's surface rather, well . . . bumpy. In order for there to be
some kind of standardization for meteorological data collection, all data
is related to sea level, which is basically constant. Through various
equations (which we'll discuss in later sessions), data, such as pressure,
is converted to an equivalent sea level pressure. Therefore, it is
important to know standard measures of parameters at sea level in order to
make a comparison.
The standard air density at sea level is about 1.2 kilograms (kg) per
cubic meter. The standard air pressure at sea level (known as sea level
pressure) is 1013.25 mb (101.325 kPa). The standard temperature for sea level is 15
°C (59 °F). By using these values as standard for the
atmosphere, we can compare all other measured values at various locations
around the world and make a valued comparison. It means nothing to see on
the news that the pressure outside is 940 mb (94.0 kPa) if you do not know the
standards. If you do, you will realize that you are probably in the middle
of a ferocious storm. However, if you saw a pressure of 1020 mb (102.0 kPa), you would
know that you were probably having clear skies.





 The Shodor
Education Foundation, Inc.
The Shodor
Education Foundation, Inc.