HOME
Course Chapters
Calculator Fundamentals
Mathematics Review
Basic Concepts
Advanced Concepts
Section Tests
Pre-test
Post-test
Useful Materials
Glossary
Online Calculators
Redox Calculator
Kinetics Arrhenius Calculator
Thermodynamics Calculator
Nuclear Decay Calculator
Linear Least Squares Regression
Newton's Method Equation Solver
Compressibility Calculator
Units Conversion Calculator
Nomenclature Calculator
Related Information Links
Texas Instruments Calculators
Casio Calculators
Sharp Calculators
Hewlett Packard Calculators
Credits
Contact Webmaster
|
Practice Problem 4 Solution:
If the density of mercury is 13.534 g/cm3 and
you have 62.5 cm3 of mercury, how many grams, moles,
and atoms of mercury do you have? (Mercury has a mass of 200.6 g/mol.)
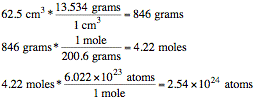
Back to Practice Problems
|

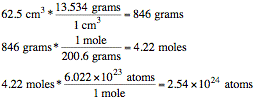
 Shodor
Shodor